

It consists of two functional groups, carboxyl and an amino.
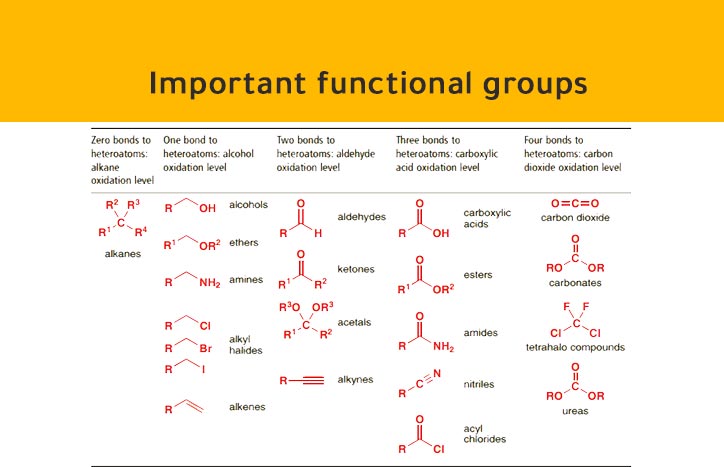
According to the priority it is an alcohol with an amino group on carbon number 3. It consists of two functional groups, hydroxy and an amino. The compound is 3-chloro-2-hydroxybutanoic acid So it is a carboxylic acid with a hydroxy group on carbon number 2 and a chloro on carbon number 3. It consists of three functional groups, carboxyl, hydroxy and chloro.Īccording to the priority the root chain of carbons must include the carboxyl group. *** Note that since the parent molecule is an acid and not an alkene the last "e" in the ene is removed. So it is a carboxylic acid with a double bond on carbon number three. It consists of two functional groups, carboxyl and a double C=C bond.Īccording to the priority the root chain of carbons must include the carboxyl group. So it is propanoic acid with a hydroxy group on carbon number two. Is it an alcohol or is it an acid? According to the priority list the root name is a carboxylic acid. It consists of two functional groups, hydroxy and a carboxyl group. Now looking at the molecule on the right we can see that the amino functional is clearly not the priority, so we use the prefix amino to derive the name 2-aminopropanoic acid.Ĭonsider the compound shown on the right. Since the amino functional group is the priority then we use the suffix amine to derive the name propan-1-amine. So the name of this molecule is butan-2-olĬonsider the molecule shown on the right. In a molecule where the hydroxyl group is a priority the suffix ol is used. So the name of this molecule has the prefix hydroxy and is given the name 3-hydroxybutanoic acid. Coming off the third carbon is a hydroxyl group. The molecule is clearly an acid where the carbon backbone is butanoic acid. The carboxyl functional group is the priority. If the amino functional group is the priority then we use the suffix amine, however, if it is not a priority we use the prefix amino.Ĭonsider the molecule on the right. If the hydroxy functional group is the priority then the molecule is an alcohol and has a suffix of ol, but if its not the priority it has the prefix of hydroxy. The question here is when should you use the terms hydroxy or amino instead of alcohol and amine. Now let's first of all look at amines and alcohols.Īlcohols have the hydroxy (OH) functional group while amines have the amino (NH 2) functional group. The priority of the functional groups are listed below from highest to lowest priority carboxyl groups been the highest in priority. Chemistry - organic-naming compounds with more than one functional group Naming compounds with more than one functional group


 0 kommentar(er)
0 kommentar(er)
